Sales and Marketing : For Domestic : +91 8550986868
- For International Sales and Marketing : +91 8551986868
Sales :sales@anantlabs.com
Search by API Index
A B C D E F G H I J K L M N O P Q R S T U V W X Y ZImpurities available for
. Anant Labs manufacture and furnish high quality impurities for global markets. Our EP Impurities are used by API manufacturers and fully integrated pharmaceutical companies worldwide. Anant Labs offers impurities.
Total Products : 2240
(Page 1 of 94)
-
-
-
-
-
-
-
-
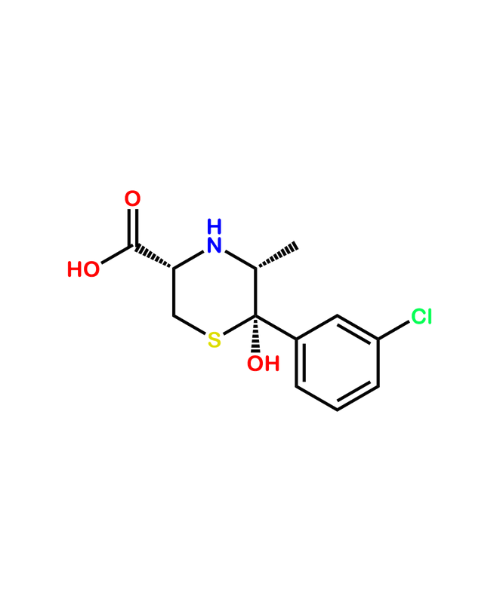
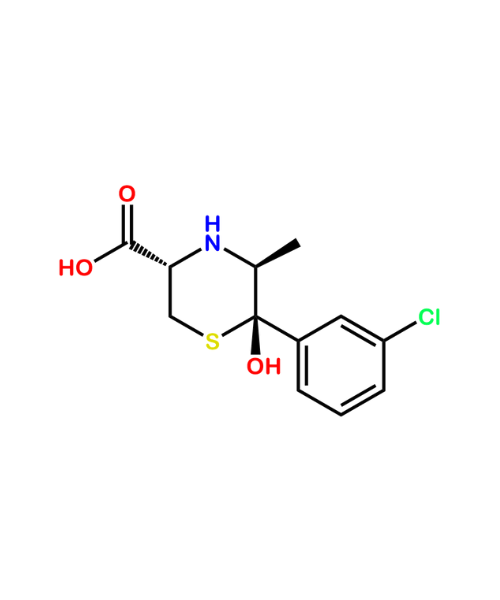
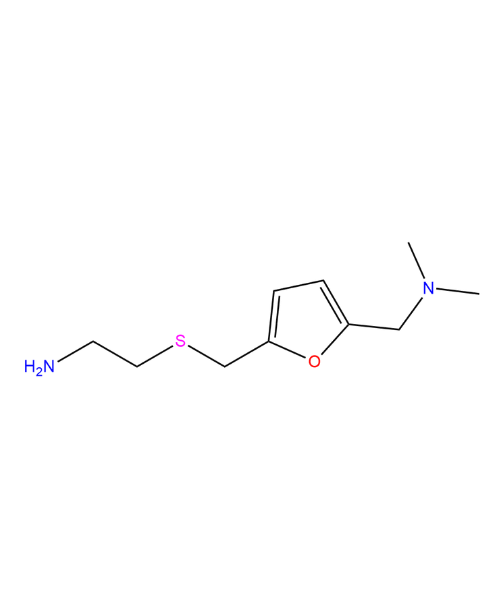
Ranitidine Related Compound A (USP)
CAT Number: ANT-RNT-04CAS Number: 66356-53-4Stock: Please Enquire -
-
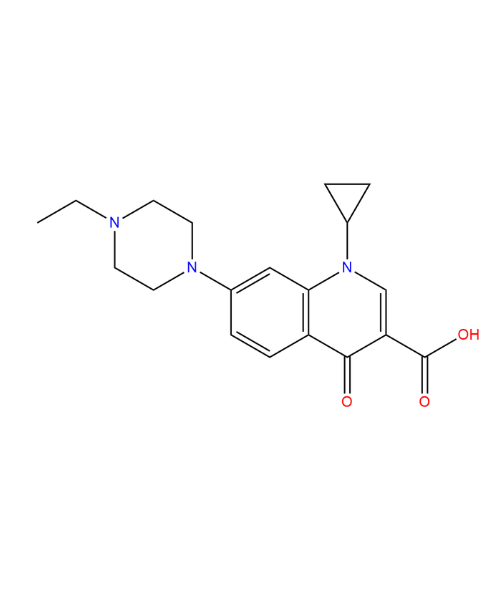
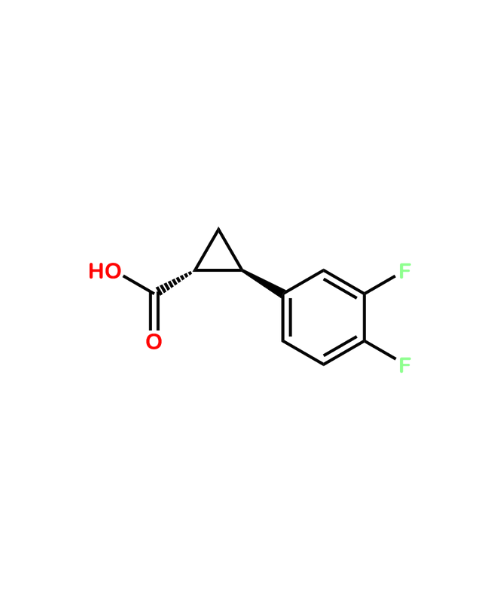
(1R,2R)-2-(3,4-Difluorophenyl)-cyclopropanecarboxylic Acid
CAT Number: ANT-TCG-33CAS Number: 220352-36-3Stock: In-stock -
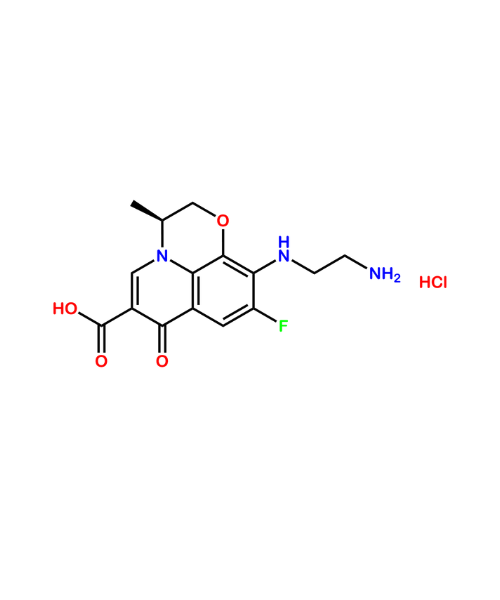
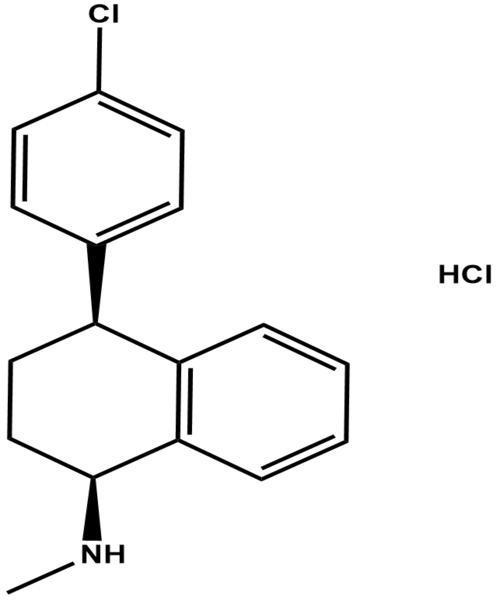
(1S)-4,5-Dimethoxy-1-[(methylamino)methyl]benzocyclobutane Hydrochloride
CAT Number: ANT-IBD-06CAS Number: 866783-13-3Stock: In-stock -
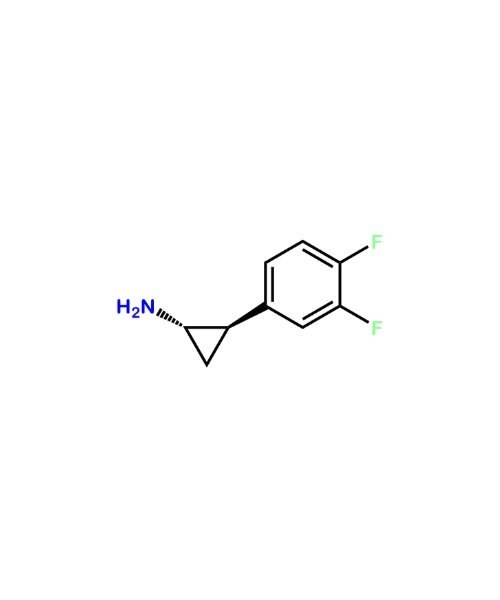
(1S,2R)-2-(3,4-Difluorophenyl)-cyclopropanamine
CAT Number: ANT-TCG-25CAS Number: 1345413-20-8Stock: In-stock -
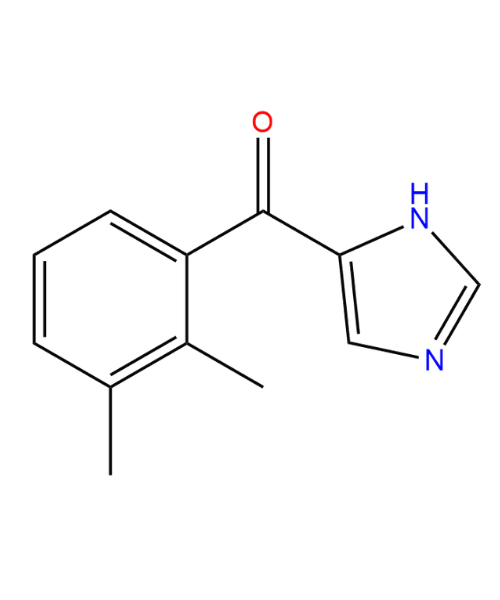
(2,3-Dimethylphenyl)(1H-imidazol-5-yl)methanone
CAT Number: ANT-DXD-01CAS Number: 91874-85-0Stock: Please Enquire -
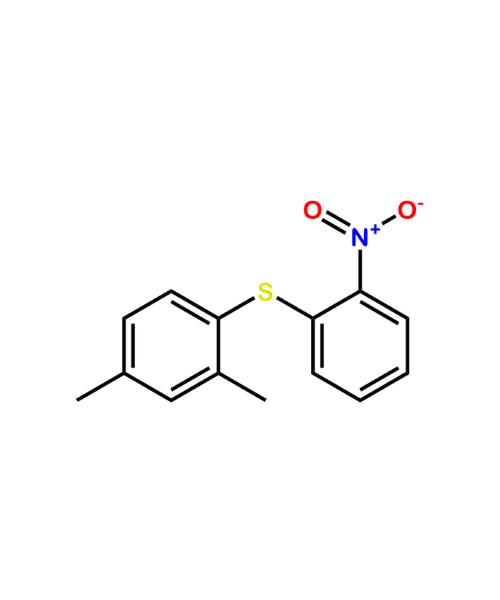
(2,4-Dimethylphenyl)(2-nitrophenyl)sulfane
CAT Number: ANT-VRT-02CAS Number: 1610527-49-5Stock: In-stock -
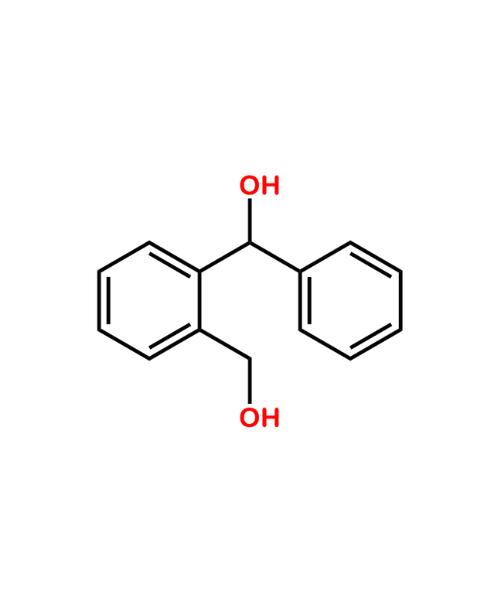
(2-(hydroxymethyl)phenyl)(phenyl)methanol
CAT Number: ANT-NFP-06CAS Number: 1586-01-2Stock: Under Synthesis -
(2-Chloro-7-cyclopentyl-7H-pyrrolo[2,3-d]pyrimidin-6-yl)methanol
CAT Number: ANT-RBC-02CAS Number: 1374639-77-6Stock: In-stock -
-
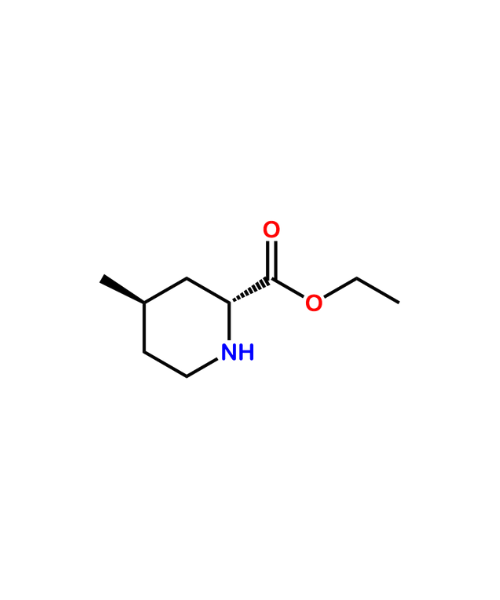
(2R,4R)-Ethyl 4-methylpiperidine-2-carboxylate
CAT Number: ANT-AGT-06CAS Number: 74892-82-3Stock: In-stock -
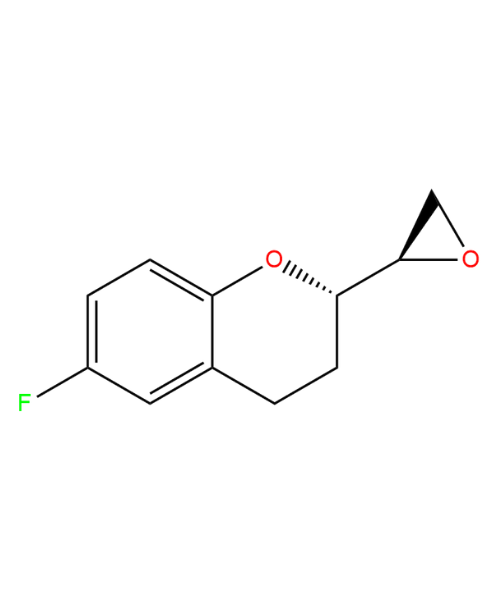
(2S, 2'R)-6-Fluoro-2-(2'-oxiranyl)chromane
CAT Number: ANT-NBV-04CAS Number: NAStock: Please Enquire -
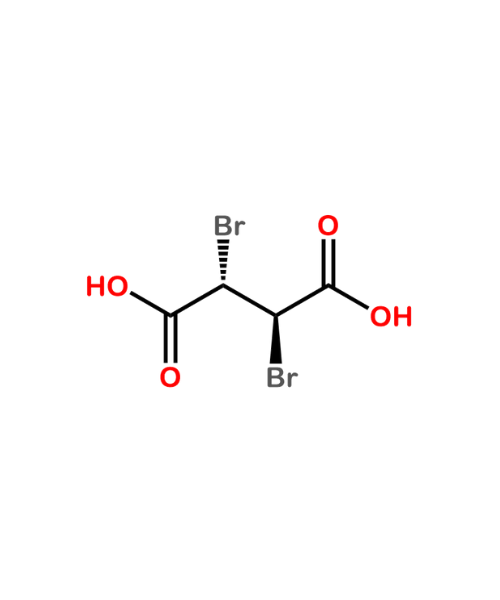
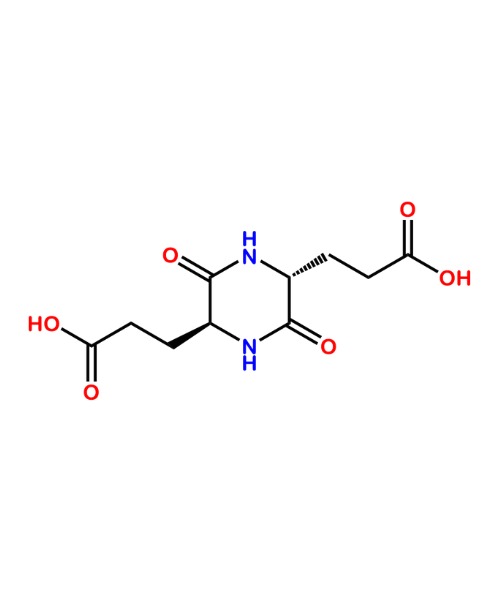
(2S,5R)-3,6-Dioxo-2,5-piperazinedipropanoic acid
CAT Number: ANT-PDT-02CAS Number: 325481-51-4Stock: Under Synthesis -
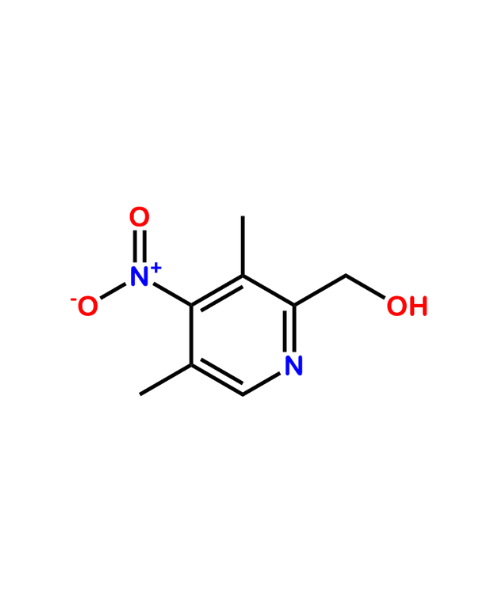
(3,5-Dimethyl-4-nitropyridin-2-yl)methanol
CAT Number: ANT-OMP-24CAS Number: 149082-03-1Stock: In-stock -
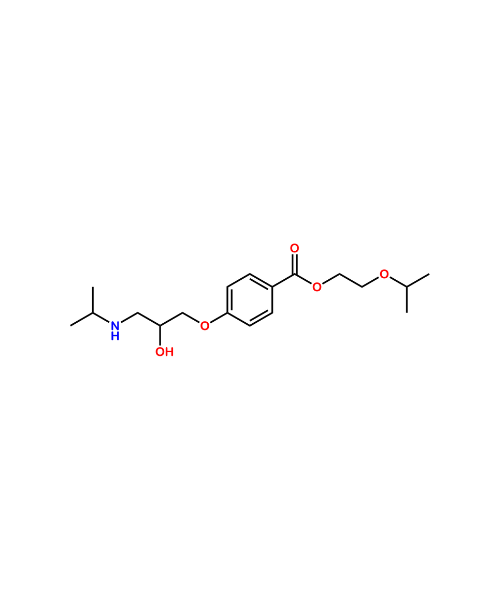
(3R,4R,5R)-4-(1-Ethylpropoxy)-3-hydroxy-5-[(methylsulfonyl)oxy]-1-cyclohexene-1-carboxylic Acid Ethyl Ester
CAT Number: ANT-OST-13CAS Number: 204254-94-4Stock: In-stock -
-



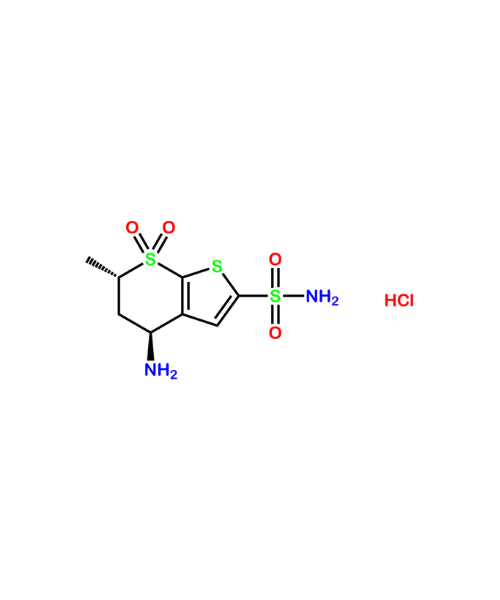
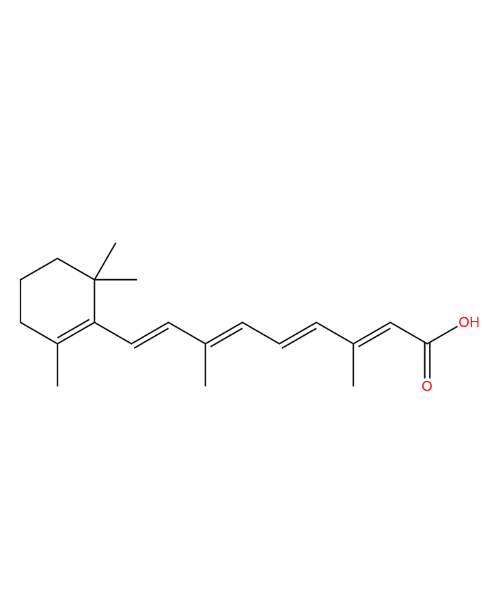
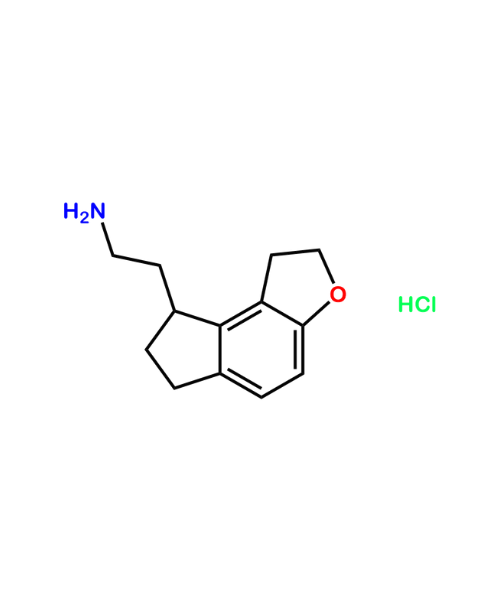
![(1S)-4,5-Dimethoxy-1-[(methylamino)methyl]benzocyclobutane Hydrochloride](https://anantlabs.com/cms/attachment/ANT-IBD-06.png)
![(2-Chloro-7-cyclopentyl-7H-pyrrolo[2,3-d]pyrimidin-6-yl)methanol](https://anantlabs.com/cms/attachment/ANT-RBC-02.png)
![(3R,4R,5R)-4-(1-Ethylpropoxy)-3-hydroxy-5-[(methylsulfonyl)oxy]-1-cyclohexene-1-carboxylic Acid Ethyl Ester](https://anantlabs.com/cms/attachment/ANT-OST-13.png)